Dye Dilution Fundamentals Explained
Wiki Article
The Single Strategy To Use For Dye Dilution
Table of ContentsWhat Does Dye Dilution Mean?Dye Dilution - QuestionsAbout Dye DilutionDye Dilution Fundamentals ExplainedSome Known Questions About Dye Dilution.Dye Dilution for DummiesDye Dilution Can Be Fun For AnyoneHow Dye Dilution can Save You Time, Stress, and Money.What Does Dye Dilution Do?
Serial dilutions are made by making the exact same dilution action over and over, utilizing the previous dilution as the input to the next dilution in each step. Since the dilution-fold is the very same in each action, the dilutions are a geometric series (continuous proportion in between any type of surrounding dilutions). For instance: Notification that each dilution is three-fold about the previous one. 
Some Ideas on Dye Dilution You Need To Know
This avoids bunching many of the punctuate at one end and also having just the last factor way far down the scale. Before making serial dilutions, you need to make rough price quotes of the concentrations in your unknowns, and also your unpredictability in those estimates. If A280 says you have 7.7 and also 7 mg/ml. That indicates you require to cover a ten-fold variety of dilutions, or maybe a bit extra to be sure. If the half-max of your assay occurs at concerning 0. 5 mg/ml, after that your minimum dilution layer is (700 mg/ml)/(0. 5 mg/ml) = 1,400. Your optimum is (7000 mg/ml)/(0.
The Best Guide To Dye Dilution
To be risk-free, you could want to cover 1,000 via 20,000. Generally, prior to creating a dilution collection, you require to decide: What are the most affordable and also highest focus (or dilutions) you need to test in order to be specific of discovering the half-max? These determine the series of the dilution series.It is a lot less complicated to opt for 2-fold dilutions and provides regarding the same outcome.) You need to make a 1/1,000 dilution to begin with. Then you require to serially thin down that 2-fold per action in five actions. You can make 1/1,000 by including 1 microliter of sample to 0.
The 2-Minute Rule for Dye Dilution
Since you can not gauge 1 microliter (or even 10 microliters) accurately with ordinary pipeters. Make 3 serial 1/10 dilutions (0. 0 ml of the beginning 1/1,000 dilution to 1.0 ml from that dilution (leaving 1. And also so forth for 3 even more serial dilution actions (providing 1/8,000, 1/16,000, and 1/32,000). 0 ml of each dilution.
6 Easy Facts About Dye Dilution Described
Water is the most abundant part in the human body consisting of concerning 60% of body mass in the referral guy. Because it is mostly discovered in the fat-free body in a relatively consistent amount, evaluation of body water has been of interest as an approach of body structure analysis for nearly 100 years.Water's characteristic as a single molecular varieties offers itself to making use of the dilution principle, which in its most basic kind, states that the quantity of the component is equal to the amount of isotope included in the component split by the focus of discover this info here the isotope because part. In 1915, the dilution principle was initially made use of in the research study of body composition when using a red dye to gauge the plasma volume was theorized.
Everything about Dye Dilution
Using a mathematical technique, a sensible estimate was made to calculate the volume of plasma in which the dye was initial weakened. Following this examination and using the very same principle, tracer material was injected intravenously and permitted to reach an uniform distribution, as well as from the dilution achieved at balance, the constituents of the body were determined.Tritiated water was very first described by Speed et al. as an isotope for determining TBW - Dye Dilution. The primary advantage of using tritium (3H), the contaminated isotope of hydrogen, is that it is conveniently available as well as quickly assayed by scintillation checking. On the various other hand, a large quantity of tritiated water have to be carried out to acquire ample accuracy, eliminating its use in cases where using radionuclides is limited.
An Unbiased View of Dye Dilution
Greater technological mistakes have actually been found using the infrared strategy. When making use of isotope dilution, particularly deuterated water, two body liquid examples from pee, blood, or saliva are gathered: one right before administration of the deuterium dose to figure out the natural history degrees and the 2nd after enabling sufficient time for infiltration of the isotope.There are 4 fundamental presumptions that are inherent in any type of isotope dilution technique. The isotope is distributed only in the exchangeable swimming pool. None of the typically used isotopes are distributed just in water. Tracer exchanges with nonaqueous particles are very little, as well as as a result, the volume of distribution or dilution space of the isotope can be identified, albeit slightly better than the water pool.
The Main Principles Of Dye Dilution
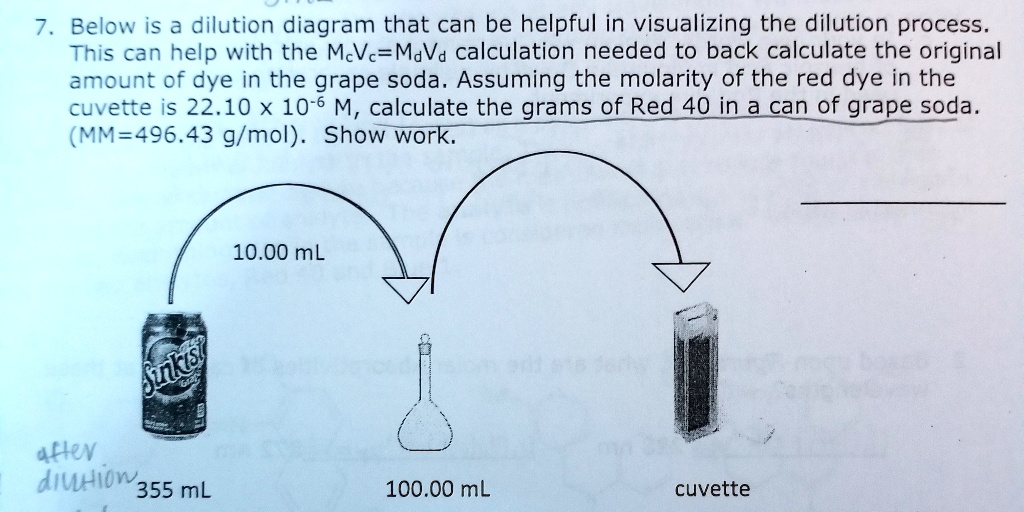

Still, go now it is necessary learn this here now to consider gaps after tracer administration. Three voids are suggested after the dose when pee is used as the biological example. The tracer is not metabolized during the equilibration time. Body water remains in a continuous state of change. In pleasant climates, the average fractional turnover rate in grownups is 8% to 10% daily.
An Unbiased View of Dye Dilution
The inputs are stabilized by an outcome of water in the kind of pee, sweat, breath water, or transdermal dissipation. This constant turn over has caused two methods when evaluating TBW: the plateau technique and also the back-extrapolation, or slope-intercept, technique. For body composition research, the plateau approach is the typical method.Report this wiki page